
Here’s Why the Jury’s Still Out on Lenire
By Hazel Goedhart, Director of Tinnitus Hub
You’re suffering from tinnitus and you’ve heard of this new treatment called Lenire. You’re wondering if it might be right for you. You might have found the Lenire thread on our support forum Tinnitus Talk but gotten discouraged at the thought of reading 7.000+ posts. You just want a quick, easy and, most importantly, independent assessment of this device. That’s why we compiled this article for you.
We cover:
- What Is Lenire?
- How to Get Lenire?
- How Was Lenire Tested?
- Does Lenire Work?
- Is There Any Independent Study on Lenire?
- Should You Buy Lenire?
- Help Us Continue to Inform You
- Learn More About Lenire
What Is Lenire?
The tinnitus treatment device called Lenire® was launched by Neuromod Devices in June 2019. It has been the only serious attempt at a new tinnitus treatment of the past few years – ignoring of course the plethora of obvious scam products hitting the market every day.
Lenire is based on “bimodal neuromodulation” technology, which means stimulating two neural pathways simultaneously. In the case of Lenire, we are talking about sound stimulation through headphones at the same time as the trigeminal nerve being stimulated through electrical pulses to the tongue.
How to Get Lenire?
Lenire is only available by recommendation from an appropriately qualified healthcare professional. The device is currently offered through clinics in Ireland, Germany and Belgium. Whereas several visits to one of the clinics were previously required, due to the impact of COVID-19, Neuromod has been offering remote appointments for residents of Ireland and other EU countries since June 2020 from its clinic in Dublin, thus removing the need to travel.
Neuromod applies a pre-treatment screening, so beware that you may be told during/after your first appointment that you are not eligible (for instance due to severe hearing loss).
The device is priced at a steep EUR 2.500 – which is currently not covered or reimbursed by any public healthcare schemes.
How Was Lenire Tested?
The tinnitus community has long awaited the release of clinical trial data to support Neuromod’s claims about Lenire’s benefits. A group of researchers published their analysis of the company’s TENT-A1 trial in Science Translational Medicine (STM) – a highly regarded and peer-reviewed academic journal – earlier this month. You can find the article here.
It’s clear from the STM article, and the previously published trial protocol, that we are dealing with a clinical trial where the methodology is clearly described, the sample size is adequately large, outcomes are reported in detail, and long-term follow-up of participants was conducted. Such rigor has been sorely lacking in many tinnitus studies for the past few decades; hence, we applaud Neuromod for its diligence in this regard. However, we also identified some weaknesses in study design and reporting, which we will discuss below.
Does Lenire Work?
This is of course the million dollar question. Neuromod summarizes the results of their trial as follows: “86% of treatment-compliant participants experienced an improvement in tinnitus symptoms after 12 weeks of treatment.” This sounds promising at face value. But we have some caveats to the clinical trial results as reported in the STM article:
- 86% refers to participants that showed ANY improvement, no matter how little. It has been demonstrated that natural improvement occurs for most people with tinnitus over time, so some of those 86% might have improved anyway without the device. See this study for instance, which attempts to quantify the degree of natural improvement for tinnitus.
- Furthermore, an improvement of a few points on the Tinnitus Functional Index (TFI) or Tinnitus Handicap Inventory (THI) scale is not very meaningful. One could expect to see at least 50% “improvers” just from small variations in before/after questionnaire responses, unrelated to any kind of treatment. For both the TFI and THI scales ‘clinically significant’ improvement thresholds have been defined – reported here for TFI and here for THI. Although Neuromod has previously spoken of the minimally clinical important difference (MCID) outcomes of their clinical trials, for instance in the Tinnitus Talk Podcast, the STM article unfortunately does not report what percentage of participants experienced such a meaningful improvement.
- The article also does not report what types of patients were more likely to benefit. Is tinnitus duration a factor? Is hyperacusis a factor? (Neuromod has previously stated that it is.) These questions remain unanswered until further analysis is done on the clinical trial data, which apparently is planned for a future paper.
- Lack of placebo control: It is well-documented and proven many times over that ANY intervention (no matter how useless) will lead to SOME improvement simply because of the participant’s expectations or because they are given personal attention by an authority figure. In this study, however, no placebo group was used to correct for that effect. Neuromod CEO Ross O’Neill has previously stated in the Tinnitus Talk Podcast that designing a placebo for a bimodal stimulation treatment is challenging. However, Dr. Susan Shore, who is also working on a bimodal stimulation treatment, has designed a placebo version of her treatment, as described in this paper, which she also talks about in our video interview with her.
- The STM article does not report on whether Lenire resulted in a reduction of tinnitus loudness. As tinnitus expert Dr. Richard Tyler stated: “You have tinnitus, and you have your reactions to tinnitus. Those are two different things. If you’re going to try and decrease the tinnitus, then you should be measuring the tinnitus.” Neuromod has previously spoken of their efforts to measure loudness with Minimum Masking Levels (MML) but those results have not (yet) been reported.
That might seem like a lot of criticism, considering that there are other tinnitus treatments on the market with much poorer supporting evidence. That is true, and trust me, we would love to conduct comprehensive reviews of all those treatments (and maybe we will). But as a small volunteer organization, we have to focus on what is currently new or generating interest. What is more, we are confident that the last word has not been spoken on bimodal stimulation. More data will come out, new players will join, treatments will be tweaked. So, we certainly hold out hope, but for now would not consider Lenire a breakthrough.
Is There Any Independent Study on Lenire?
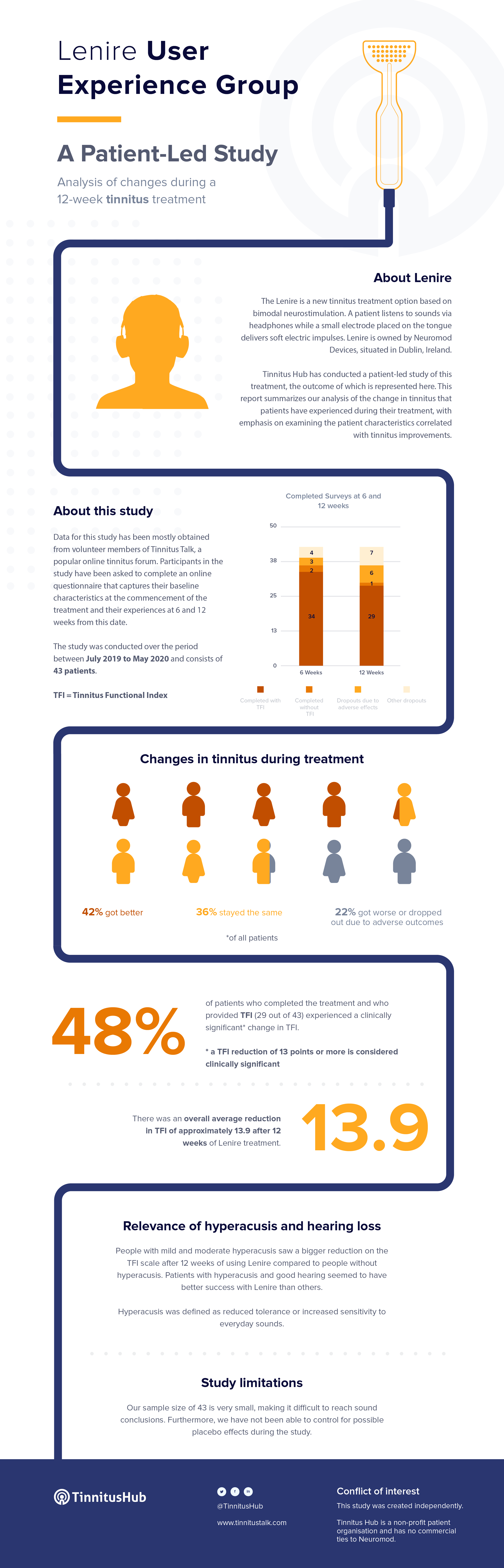
Tinnitus Hub has conducted its own, independent, patient-led study on Lenire.
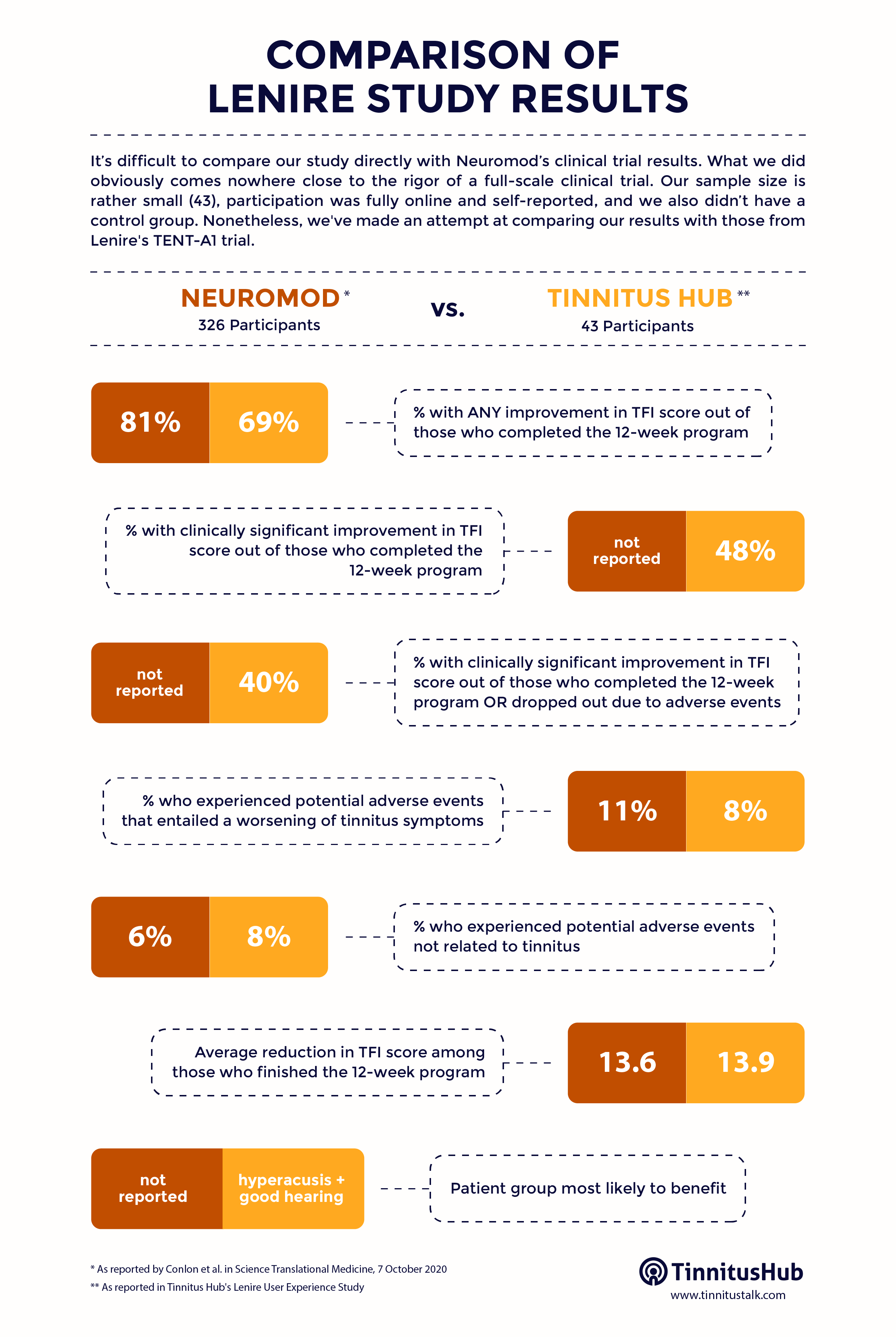
Please check here (PDF) for more details on the calculations behind this table.
Should You Buy Lenire?
There is no straightforward answer to that question. In our opinion – based on the currently available evidence – Lenire is not proven to be meaningfully more effective than (a combination of) existing treatment options such as sound therapy, hearing aids and counselling. We await and welcome more evidence on the efficacy of the device, and in fact Neuromod has assured us that more evidence is coming.
Nevertheless, it is clear that Lenire certainly might benefit some tinnitus patients; so, you will have to decide for yourself whether it could be right for you. Consider the following:
- If you are hoping to be cured from your tinnitus, beware that this is very unlikely to happen with Lenire, but you might experience relief from your tinnitus in terms of distress (and maybe also loudness, but evidence on this is not yet published).
- If you suffer from hyperacusis and you have no (perceivable) hearing loss, you are more likely to benefit* from Lenire, according to our study.
- Ask yourself whether the potential benefits outweigh the financial costs and potential risks? Only you can make this judgement for yourself, based on your personal situation, the evidence presented so far, and the advice of your doctor.
If you decide to give Lenire a try, don’t forget to tell the rest of us how it worked for you!
* Please note that we are talking about potential benefits regarding your tinnitus symptoms; there is no evidence that Lenire improves hyperacusis in any way.
Help Us Continue to Inform You
This blog and the additional resources below are the result of literally thousands of hours of volunteer labor conducted by people who suffer from tinnitus, just like yourself. Please help us to continue to deliver the independent resources you seek, by making a donation of any size.
Learn More About Lenire
What do Lenire users say?
- Read their first-hand experiences in this thread!
Tinnitus Hub’s Independent User Experience Study – June 2020
- 1-minute video summarizing our results
- Easy-to-read infographic presenting our key results
- Brief results report (8 slides)
- Full results report (73 pages)
Our interviews with Neuromod’s CEO Ross O’Neill
- Tinnitus Talk Podcast Interview with Neuromod – November 2019
- Tinnitus Hub Meets Neuromod (Q&A) – November 2018

62 Comments
Thanks for putting this all together! All the information is much easier to digest this way.
Thanks for the nice feedback, Kendra, that’s good to hear!
I spent a big part of my savings on Lenire last year, and it did nothing for me. I think I’m actually a little worse now, I get this sensitive feeling in my ears when I’m out and about.
I’m also worried about the Bluetooth connection. They should have made the device wired.
I haven’t given up on bimodal stimulation yet. Waiting for Dr. Shore’s device from University of Michigan.
Me too!
I’m not as active on the forums anymore, but my tinnitus is as bad as ever after 18 months of diligent Lenire use. I’ve sent an e-mail today to the University of Michigan asking to be added to their mailing list regarding their version of this technology. Can you imagine how much you’d be willing to pay if someone said ‘guaranteed to work or your money back’?
Here’s to a quieter 2021.
I too would like to be part of trying Dr. Shore’s treatment.
Thanks for your candid and truthful reporting. That’s a little slice of Heaven in the world we live in today!
Thank you for the informative summation! Keep up the good work.
I wonder when Lenire could see US launch? FDA is taking their sweet time. Not that I would be trying this… feels like a beta product.
This is the kind of content British Tinnitus Association and American Tinnitus Association should put out too.
MAD RESPECT FOR THE TINNITUS HUB TEAM!
Thanks for the kind words; that’s the kind of stuff that keeps us going! 🙂
The BTA have been banging on for ages about a ‘biobank’ for tinnitus. What’s stopping them doing it? It’s not like they don’t know where to find 100s / 1000s of tinnitus sufferers.
Nice review. Thanks for your time.
Great balanced analysis, thanks so much. I’m an audiologist and have the same concerns with the current study (placebo and definition of “improvement”). I am bookmarking your site!
Very helpful and succinct summary Hazel. Thank you.
Let me know when they can repair or re-grow the microscopic hair cells in the cochlea. Then I’ll have hope a *real* treatment is at hand.
Of course, by then my great (to the tenth power)-grandchildren will be living on a planet thousands of light years from what’s left of earth.
In other words, I don’t see this happening in my lifetime.
Hi Chuck,
Tinnitus has all to do with the brain, hearing loss is just a trigger in my point of view, where parts of the brain lose control of signal processing. I haven’t got any hearing loss, my tinnitus is caused by a clogged ear after a cold. Just another trigger. That’s why it’s such a difficult condition to cure. Take care and all the best.
Kind regards,
Frans
Chuck, I think you have it – perfectly!
Great summary. Thanks Hazel.
They asked me to have Lenire treatment in Germany. But I rejected that since there is not a lot of evidence it’s working. I don’t want to spend the money for nothing.
Today there will be a Webinar with Professor Lenarz and Professor Langguth about the “promising results” (as it is described) of the tinnitus therapy with Lenire. I’m looking forward to attend this. But I assume it is more promotion and marketing.
Keep up the good work and take care,
Martin69 from Tinnitus Talk
Why don’t we fund four tinnitus sufferers that have differing variations and symptoms of the ailment and test the outcomes ourselves? Cost would be €10,000 EUR.
We would then have factual information and be able to make a decision based on facts.
Thank you!
I have had tinnitus since my stroke 10 years ago. This is very interesting to me. I have used some medication but no help.
I use an ear vibration device when it is real bad. I get a little help from that. Nothing like holding your ears and crying for the tinnitus to stop.
When do you think Lenire will come to the USA?
I tore the ligaments in my rotator cuff. I had a lot of pain for three months. I think it triggered the tinnitus.
How would that be possible?
I have just ‘acquired’ tinnitus last month – immediately after an audiology test for possible hearing aids.
Is there anything in the literature that suggests this was not just a coincidence?
I don’t know about the literature on the matter, but I can tell you that my wife also started suffering from tinnitus (or at least noticing it) after an audiology test in February of last year to see if she had labyrinthitis (turns out she doesn’t). Her doctor didn’t seem to care. Since then she’s tried a bunch of different treatments and medications to no avail. I hope you do find relief somehow, and if you do, please reply to this post.
Me too!
About twice a year the Veteran’s healthcare system thinks I need to have a hearing test. And it takes 1-2 weeks for my tinnitus to calm down afterwards. I don’t see the point, of repeating a test that shows the same results?
I tried the “Lenire” device for two years, never missed a day. It cost me a lot of money and had no effect whatever with my tinnitus.
Can you provide more info such as the possible cause of your tinnitus and the type of tinnitus you have? That would be helpful, so we can see if there’s only certain groups who respond or don’t respond.
Hello Bill!
Would you be willing to speak with us or email our 20-year-old son, who has level 4 tinnitus and is considering flying from the USA to Germany very soon to purchase a Lenire device and seek treatment from the device.
We have used every option that the USA has to currently offer medically. We found your post on Lenire very disheartening. Any feedback would be greatly appreciated.
Thank you for the report. It’s great to read other people’s opinions.
My poor husband has suffered from tinnitus for a few years now but it was manageable until this weekend. He said it was intolerable and he was in so much pain. He’s in construction and in his 40s. He had a hearing test a week ago. Reading some previous posts this may have triggered the flare up. We are only starting the path to finding ways to help him.
Just found your Lenire article today and it’s brilliant. So thank you. I will press the donate button.
Impressed by your objective critique.
I also used Lenire for around 2 months. At the beginning, it helped, however at some point it did the opposite effect to the point that I am really struggling at the moment. I dropped the treatment and regret so much having used it. I can not sleep at all. I’m in a very bad state.
I tried Lenire for several months. It cost €2,500. No sign of help. In fact, during my time using it, I got tinnitus in the left ear, which I never had before. Was it a cause? Not definitive, but I previously had tinnitus only in my right ear for 10 years. I am one where my tinnitus to date has created zero hearing loss. I’ve always had good hearing.
I would not recommend Lenire. Plus, the follow-up care is not good. Big thumbs down.
Stay positive though.
I have suffered from tinnitus for over 20 years. The condition seems to get worse year on year and now I focus on it more and more throughout my waking day.
Any suggestion of treatment that might alleviate the symptoms sounds too good to be true because I have seen so many remedies over the years that come to nothing.
With Lenire there comes a great cost – anything between £3K-£5K and I am reluctant to throw hard earned money that is unproven and might not work at all.
What happens if it doesn’t work? I won’t get my money back. Are there options to sell the equipment back to the clinic? How far will I need to go in the treatment before I realise if it won’t work?
Hoping for a miracle soon! ❤️?
Wow! It’s very refreshing for people to take a scientific approach to medicine these days, even taking account the social desirability bias (participants wanting to “perform”).
I stumbled across this article after seeing Lenire advertised and thinking “sounds interesting, but I’m sceptical”
Have just subscribed to your blog 🙂
I tried Lenire in 2021. My tinnitus spiked after 6 weeks of treatment. They told me to stop and then resume which I did. This treatment sent me to a very dark place until I stopped. It took a good 6 months for my tinnitus to return back to baseline.
Very disappointing after the hype and money spent, including 2 trips to Dublin.
I got tinnitus a little over 5 months ago and had read about habituation, that most people get better in 6 months to 18 months. Is that not true?
I have suffered from bi-lateral tinnitus of variable severity for 6 years now and was tempted by the Lenire device as I’m based in Ireland.
However, the price/benefit ratio seems to be completely wrong. Why should one have to pay so much for a treatment that has a relatively low success outcome? Imagine going to an optician who tests your eyes, takes €2500 off you and hands you your new pair of glasses. As they hand them over they say to you “By the way, there is a 50% chance that these will not make any clinically measurable improvement to your eyesight at all and, sorry, no refunds”.
Has ANYONE had success with Lenire? Im reading only negative comments.
ms
I have contacted my audiologist about getting Lenire. My THI is 60+, and most days are hellish.
My hearing aids are my best tool currently. Mild hearing loss, so using them for tinnitus sound therapy.
Yes, Lenire could make it worse. At this point, willing to throw the dice.
I acquired the Lenire device for 2970 EUR with high hopes of reducing my tinnitus. It’s a lot of money for a retired person. My tinnitus was the direct side effect of taking the Moderna COVID-19 vaccine which I now also regret taking. The second day I used the device, when I took my headset off, I was shocked how much louder my tinnitus was. It ruined my whole day. Normally during the day I was able to ignore my tinnitus and basically not be aware of it until things quietened down in the evening. I’ve tried to keep my eye on the end result and used the device every day, hoping to “retrain” my brain as the device is marketed for. After one month my tinnitus is so piercingly loud that now, during the day it can’t be ignored and it really interrupts my concentration. I’m actually scared how loud it’s become.
I blame the Lenire device for stimulating my tinnitus in a way that has basically backfired.
Has your tinnitus returned to pre-Lenire levels? I am worried because I had the same experience, and I am hoping to get back to a livable baseline.
This same thing happened to me. I used Lenire for 4 months twice a day. My tinnitus is louder than when I started. I had to stop using it. I would warn anyone from trying it. I paid $3800 with a no return policy.no-return
I have started using masking hearing aids, which cost $3200, but so far, they have had very little effect. It seems tinnitus sufferers are at the mercy of all these so-called remedies.
Glad to have found Tinnitus Hub, and being able to read all the comments. Not one person reports that they got any degree of relief from Lenire. I like OldDubliner’s analogy with optics! I’m on the Lenire “waiting list” but before I do a single thing with them, I’m going to have to see much better results.
I’ve had loud tinnitus in both ears for years. It’s a combination of very high, pulsing ringing and a continuous buzzing at a different pitch. I can’t remember when I was able to sit in silence. I’m not a high-strung person, so I’m always able to stay calm. 95% of the time I’m able to ignore it. When I do find myself focused on it, I pretend that it’s a field of crickets, and then find something to distract my attention.
I saw an audiologist for the first time a week or so ago, and the only thing they offered was hearing aids, which apparently generate a counter-wave that neutralizes the tinnitus. But I have watched my husband struggling with hearing aids for the past 5 years, and I’m really reluctant to get into that!
I used the Lenire device for the 12-week period plus an additional 2 weeks for good measure. It did nothing for me. In fact, it made it worse and I am hopeful that when I stop the additional weeks – I will return to my “before Lenire” level of discomfort.
I was told by the tech that I am one of those people whose brain is so forceful that nothing will trick it into ignoring anything. Which begs the question – doesn’t everyone who spends this kind of money for relief have a brain that can’t be fooled? Of all the people I know who have tinnitus, very few are bothered much by it. First of all – are they all stupid? But more importantly – that leaves the rest of us so bothered by it to seek out any and all treatments. So if only us “brilliant” sufferers sign up – how did they get an 86% efficacy?
Just sayin’.
Agree! I’m not sure if I want to go down that road with this treatment.
Have you returned to your pre-Lenire levels? Lenire made my tinnitus much worse, and I’m scared that I’ll never get back to my pre-Lenire livable baseline.
I’ve been suffering for 7+ months now. It’s unbearable. I am a shadow of the person I once was. I am highly skeptical in general so never had much hope for Lenire, but part of me is desperate to find something to hang hope onto. Guess there’s still just nothing… surprise surprise.
Was there not more evidence released since this was published?
I just purchased Lenire for $4000 2 days ago. I have used it twice for 30 minutes each time. My tinnitus is louder now than ever and has woken me up in the middle of the night both nights. I am afraid to use it anymore. Hopefully I can get back to where I was before using Lenire. Extremely disappointed with this product. Not going to put myself through hell to maybe get some relief, because from the sounds of these reviews it probably makes things worse.
I tried Lenire upon the recommendation of my audiologist. $3500 plus tax. He said it would take 6-12 weeks to notice anything. 6 weeks, nothing. 12 weeks, nothing. I went back to him and he said “oh it will take 6 months to a year”. 6 months came and went and guess what happens after 6 months? The piece you put on your tongue quits working, to the exact day of 6 months. So the whole unit is worthless UNLESS you want to buy a new mouth piece for $375 plus tax. I quit using it, which is the reason I’m here trying to find anything that will work. It’s unbearable.
Has anyone tried taking Magnesium supplements? I just ordered some as it was suggested by my cardiologist.
I have an appointment scheduled with Lenire in January but now wondering if I should cancel it after reading the comments. I have had tinnitus for over 10 years and yes some days are worse than others.
Wow—I’m glad I found this site. I am Canadian, and nothing much is being done in Canada for tinnitus other than a sound therapy study in Hamilton, Ontario. From what I’ve read today, government funding isn’t much at all.
Lenire is in the States and was approved by the FDA in April 2023. I was thinking about going to Seattle to try this treatment. It is about a three-hour drive one way. It is $4,500 US and just over $6,000 Canadian at this time. Meh! With no guarantee it will work, that is too much. I sure don’t want to use it and have the tinnitus get worse, either.
My tinnitus started, or I first really noticed it, around spring/summer last year (2023). I thought it was due to flying to Alberta and getting my ears plugged up. Just from reading these comments, I am now wondering if there is another cause.
One of the comments mentioned regrowing the hairs in the ears. Harvard has done that with mice. Great. Now, we wait 20 years before it happens for humans. Other studies have been done on Lenire since this article. Maybe an update is in order?
Thanks so much for Tinnitus Hub, Tinnitus Talk, and your hard work.
Lenire also made my tinnitus worse. I stopped after 1 week, and that was about 4 weeks ago. I have not returned to pre-Lenire levels, and in fact, Lenire has caused immense sorrow and frustration.
My doctor told me to push through because spikes are common in the beginning, but I also experienced distressing other side effects like increased sensitivity to sounds, the feeling of brain zaps, and pain and pressure in my ears. I had moderate tinnitus with the occasional severe spikes before Lenire, but now it is worse than ever.
I doubt that the people who developed Lenire truly understand all of the potential effects that this device could have on the brain and its rewiring. We are the guinea pigs. My doctor also shamed me for dropping out of the treatment, which was unhelpful. I don’t think the local providers are given much training or have much understanding of the actual neurological mechanisms involved.
I am so upset with myself for failing to do my research, and I hope this doesn’t happen to more people. If you can live with your tinnitus as is, I would steer clear of Lenire until more is understood. The recent Washington Post article about bimodal stimulation will probably lead many more people to seek help from Lenire, and that makes me sad and worried for them.
I have been looking into the Tinearity G1 device, which was recently approved by the FDA. Does anyone know about it?
I purchased Lenire 4 months ago and have used it religiously twice a day as instructed. It has made my tinnitus worse. My tinnitus is twice as loud as when I started. I would never recommend this device.
You are paying $3800 for an MP3 player with a tongue stimulator. They never say in their literature that the device will make you worse. Please be careful and think twice before getting involved with Lenire. They will not refund your investment. Nor do they offer any help.
I have recently written to the FDA complaining about Lenire. I would recommend doing the same if you have tried the device and had adverse reactions to it.
Our community needs to stop being preyed upon by companies that sell things that do not work.
I would recommend that no one trust Lenire. It has made my tinnitus worse, and it has not gone back to where it was when I started. I would recommend writing to the FDA, which I did, and telling them Lenire is bull. They never once said your condition could get worse.
I have suffered from tinnitus, Meniere’s, and hyperacusis for as long as I can remember. I am a former rock drummer and had bad issues with my ears growing up. I recently tried an Intratympanic Steroid injection to my left ear. Oh my, it made matters worse. The tinnitus spiked and developed hiccups for about 18 hours. I had two nights of no sleep with a louder, pulsating, whooshing, and feeling off balance as if I were walking on pillows.
I thought Lenire might have been the answer, and I was willing to toss the dice for $4-5K, but after reading these experiences, I don’t think Lenire is my answer.
I think it’s kind of silly to treat something if you don’t know the cause. Tinnitus can be caused by a variety of things, including cervical spine issues. At what point do we treat the cause and not the effect? It’s hard to believe there have been no medical breakthroughs, with millions of people suffering from this life-altering, annoying, hissing, buzzing, clicking, popping, tea kettle, dentist drill effect for a lifetime.
There has to be an answer, and there has to be a cure. I’m sure COVID-19 and COVID-19 vaccines didn’t help anyone’s success rate to diminish the sound or help alleviate it altogether.
Most likely, I won’t find a cure in my lifetime, but I hope for others to follow me.
Many thanks (bedankt) Hazel for all this insightful information, as well as to all the contributors.
Thank God I found this site, because I was about to spend $4,250 in America—not including the $250 fee they charge for the initial assessment. I’ve lost so much faith in the American medical system; it’s so focused on profit.
I came across an app on the App Store called Quieten. I’ve watched some of the videos and tried some of the treatments, and it actually seems to work quite well. My tinnitus significantly decreases when I go surfing or swim in the ocean. If you don’t live near the ocean, perhaps try a saltwater pool; it might help you too.
For me, some days my tinnitus is loud for about four days straight, and then it eases off. Massage also helps, especially on the neck and scalp.
Write a Comment
The Time Is Now — Tinnitus Sufferers Take the Lead in Research
By Hazel Goedhart, Director of Tinnitus Hub It was the moment I had been working towards for years; presenting at the Tinnitus …
When Will We Finally Get a Voice in Tinnitus Research?
By Hazel Goedhart, Director of Tinnitus Hub Imagine being invited to join a group holiday, only to find out you will be travelling …
Here’s Why the Jury’s Still Out on Lenire
By Hazel Goedhart, Director of Tinnitus Hub You’re suffering from tinnitus and you’ve heard of this new treatment called …
Categories
Tags
Research Opportunities
Work with us and tap into the power of the patient population.
Read moreMore About Us
About Us Contact Us
Donate
Functional Always active
Preferences
Statistics
Marketing